Origin: a Latin derivative
meaning "Gift of the Earth."
Pure doTERRA Essential Oils Through CPTG Quality Testing
CPTG® Quality Testing
The purity of an essential oil is its most important characteristic. An essential oil that isn’t pure means you run the risk of putting germs, heavy metals, or adulterants onto or into your body, which can provoke irritation, adverse effects, or even sickness. Without an accepted standard for essential oil quality, doTERRA created its own testing process, calling it CPTG Certified Pure Tested Grade®. The CPTG process certifies that there are no added fillers, synthetic ingredients, or harmful contaminants in their essential oils that would reduce their efficacy. doTERRA even goes a step further, putting all their products and the packaging through a battery of tests to ensure a long and effective shelf-life. This protocol ensures potency, purity, and consistency batch to batch.
Before the CPTG Process Begins
Proper methods of growing, harvesting, and distilling are also crucial to maintaining purity. Poor production practices and the development of synthetic essential oil variations suggest that it is impossible to accurately identify a pure essential oil without scientific analysis. Appropriate analysis of the constituents within an essential oil is one of the most challenging and detailed aspects of quality assurance.
Knowing which of the many different species of a given plant will provide the most profound health benefits is the first step in producing the highest quality essential oil. Relying on the expertise of botanists, chemists and wellness practitioners, botanical materials are carefully selected for their natural concentrations of active aromatic compounds.
Nurturing plants in the most favorable environment and carefully harvesting and transporting plant material for processing ensures an optimal yield of pure and potent essential oils. Spanning the continents of the globe, doTERRA’s exclusive network of growers and harvesters are experts at cultivating plants specific to the essential oil industry.
The CPTG Process
The CPTG testing begins immediately after distillation with each oil being reviewed for its chemical composition. A second round of testing is carried out at our production facility to ensure that what was distilled and tested is the same essential oil as was received. A third review of the chemistry of the oil is conducted in a three-phase procedure as the oils are packaged into the bottles we use as consumers. Each of these tests confirms that the essential oil is free of contaminants and unexpected alterations during production.
The CPTG Certified Pure Tested Grade® quality protocol includes the following tests:
- Organoleptic testing
- Microbial testing
- Gas chromatography
- Mass spectrometry
- Fourier Transform Infrared spectroscopy (FTIR)
- Chirality testing
- Isotopic analysis
- Heavy metal testing
Historically, gas chromatography was sufficient to identify individual components in an essential oil. However, as more sophisticated methods for developing synthetic essential oil products formed, further validation methods were needed. Over time, additional testing methods such as mass spectroscopy, chiral analysis, FTIR Scan, carbon isotope analysis and others have been developed to more accurately identify each individual essential oil constituent.
Organoleptic Testing
Organoleptic testing involves the use of the human senses— sight, smell, taste, and touch. To expert distillers, the senses are used as the first line of quality testing to provide immediate clues to the acceptability of a product. Oil that has an unusual smell, uneven consistency, or strange color instantly tells the distiller that something is wrong. Often times, this testing is used as a preliminary quality control step before any other tests are conducted.
Microbial Testing
Microbial testing involves analyzing a batch of essential oils for the presence of bio-hazardous microorganisms such as fungi, bacteria, viruses, and mold. The process involves drawing a sample and then adding that sample to a sterile growth medium in an enclosed dish or plate. The sample is incubated for a period of time and then observed for microbial growth. This test is performed on product entering the manufacturing facility and on finished products prior to distribution to ensure that the product has not been contaminated during the filling process.
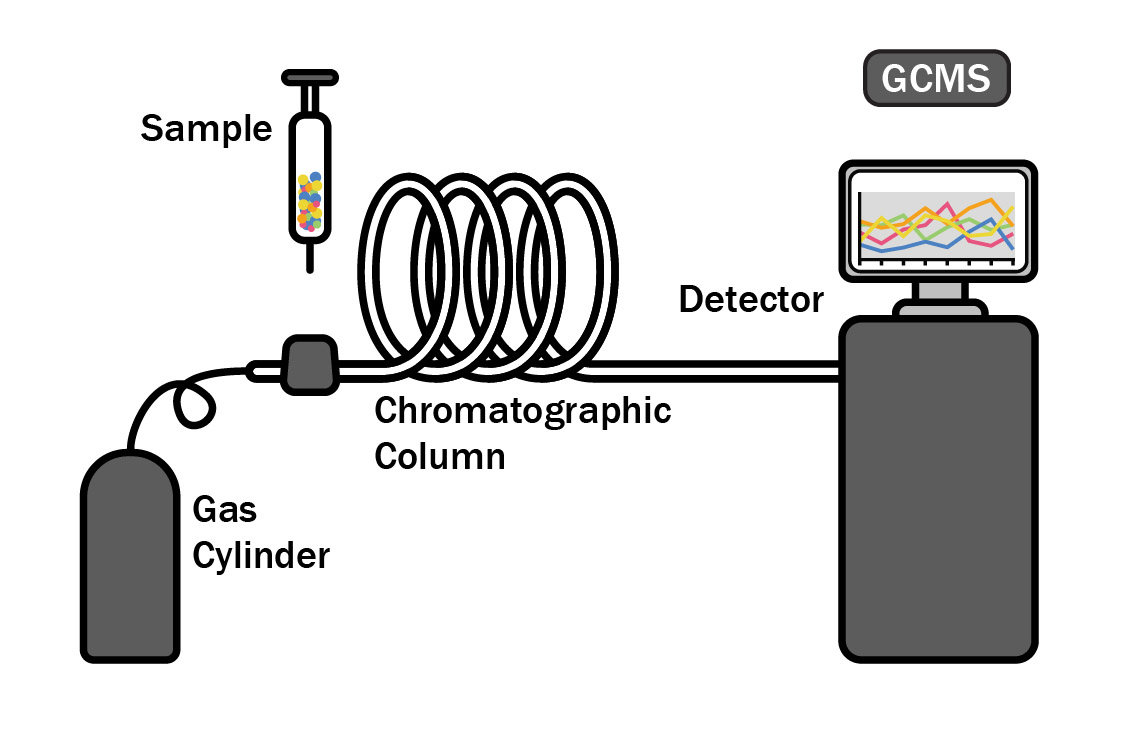
Gas Chromatography and Mass Spectrometry Analysis (GC/MS)
In Gas Chromatography, an essential oil is vaporized and passed through a long column to separate the oil into its individual components. Each component will travel through the column at a different speed, depending on its molecular weight and chemical properties, and is measured as it exits the column. Using this testing method, quality control analysts can determine which compounds are present in a test sample.
Mass Spectrometry is used together with Gas Chromatography to further determine the composition of an essential oil. In Mass Spectrometry, the constituents previously separated by GC are ionized and sent through a series of magnetic fields. Using molecular weight and charge, the amount of each constituent can be identified, providing additional insights into the potency of the essential oil.
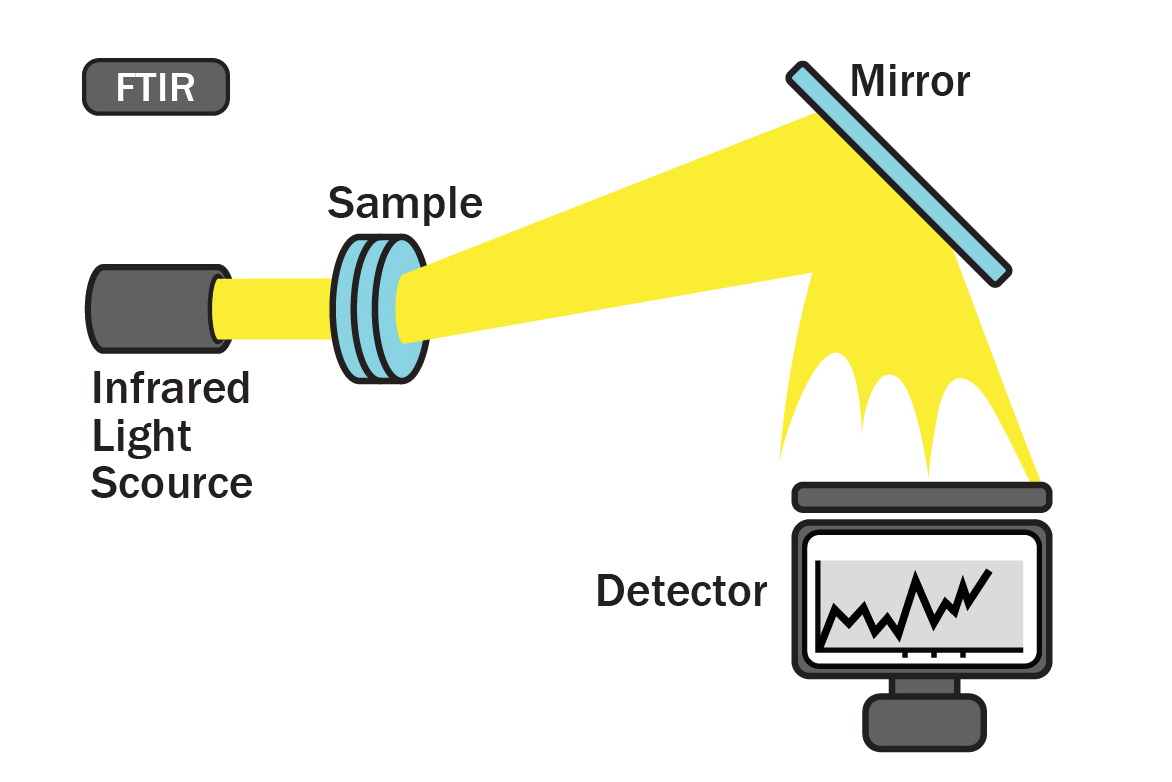
Fourier Transform Infrared Spectroscopy
Fourier Transform Infrared Spectroscopy (FTIR) is conducted to ensure the potency and consistent quality of a batch of essential oil. This testing method identifies the structural components of essential oil compounds. In an FTIR scan, infrared light of different frequencies is shined through a sample of essential oil and the amount of light absorbed by the sample is measured. The quality of the sample is determined by comparing the results from an FTIR reading to a historical database with absorption patterns of high quality samples.
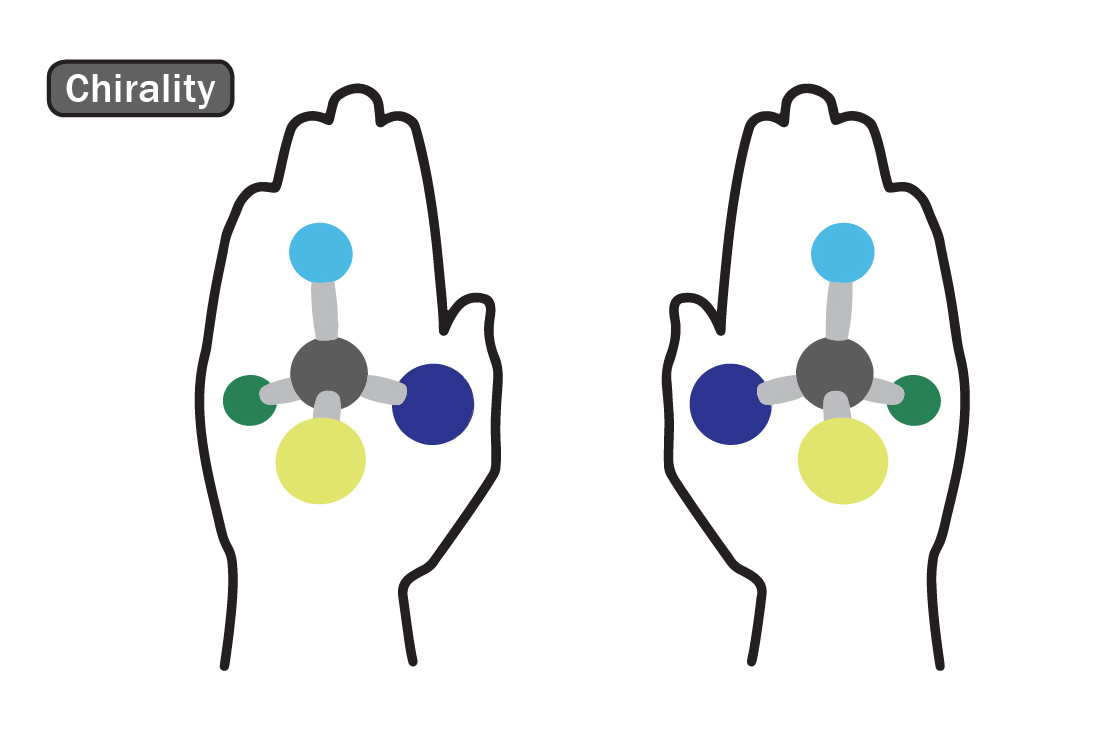
Chirality Testing
Chirality, a word derived from the Greek word “hand,” is a term used to describe the 3D orientation of a molecule. Just as you have two hands, chiral molecules exist in two forms, distinguished as either the right or the left hand. You may visualize this principle by looking at your hands; when placed side by side, they are mirror images of each other. However, when placed on top of each other, no matter how you turn them, you cannot get them to line up exactly. In molecules, each “hand” has different chemical properties, which affects their physiologic interactions in the body. One hand is produced predominantly in nature. However, in a laboratory environment, the ratio of right- to left-handed molecules is always 50/50 due to their structural similarities. The ratio of right- to left-handed constituents can be determined through a special type of Gas Chromatography. Although not commonly performed on a batch-to-batch basis, this testing method is used to ensure that no synthetic elements are present.
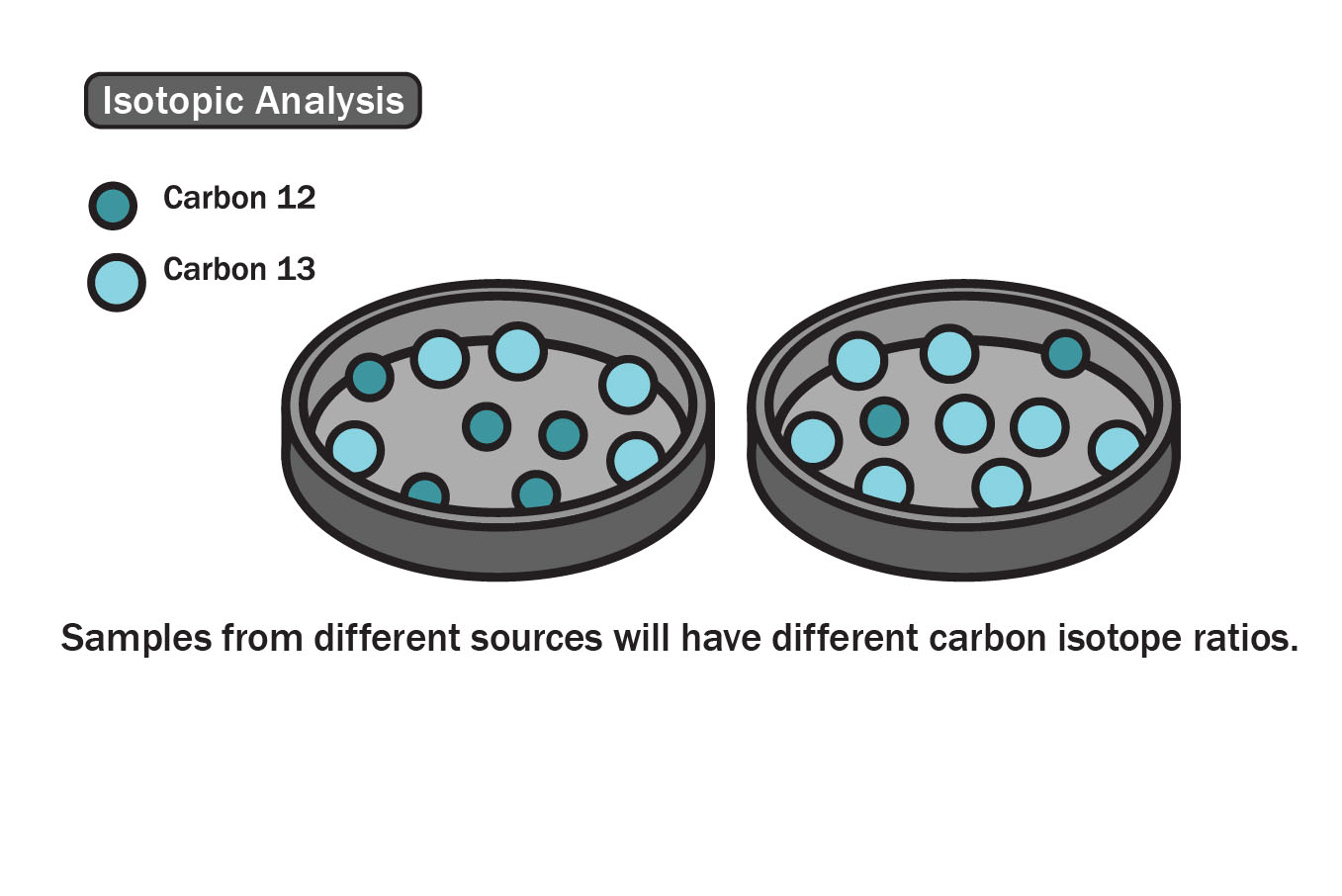
Isotopic Analysis
Matter is made up of tiny chemical building blocks called elements. Although dozens of elements exist, each one is distinct due to the protons it contains. Sometimes, an element can exist in more than one stable form if it has more or less neutrons. When this occurs, the elements are called isotopes. The element carbon exists in two stable isotopes, carbon-12 (6 protons and 6 neutrons) and carbon-13 (6 protons and 7 neutrons). Because essential oils are organic compounds, they are composed primarily of carbon atoms and will have a certain ratio of carbon-12 to carbon-13 isotopes. This ratio varies based on location around the world.
Using a special type of Mass Spectroscopy, it is possible to determine which isotopes are present in an essential oil constituent and at what amounts. If sourced from the same location, every constituent in an essential oil should have the same ratio of isotopes. If a particular constituent has an isotopic profile different than that of the other constituents, then the quality control analyst will know that the oil contains an adulteration.
Heavy Metal Testing
Heavy Metal testing shows the amount of heavy metal content in the essential oil. When properly distilled, essential oils should not contain heavy metals. ICP-MS testing uses a high-energy medium called Inductively Coupled Plasma (ICP) to ionize the sample. The sample is then run through a mass spectroscope, which separates the sample into its elemental parts and provides a reading about which elements are present and at what quantities.
Video disabled by your privacy settings




