Origin: a Latin derivative
meaning "Gift of the Earth."
- Shop
-
Our Story
- View Our Story Home
-
Who we are...
-
Who we are...
An essential oil company changing the world one drop at a time.
- About doTERRA
- Our Story
- Executives
- Science
-
-
What we do...
-
What we do...
Provide pure, high quality essential oils and products.
- About Essential Oils
- What is an essential oil?
- Why doTERRA?
- Essential Oil Safety
-
-
Why we do it...
-
Why we do it...
To empower you and your loved ones to live a wellness lifestyle.
- The dōTERRA Difference
- Co-Impact Sourcing
- CPTG® Quality Control
- Global Botanical Network
- Product Innovation
- Source to You
- Co-Impact Sourcing Projects
- Bulgaria
- Guatemala
- Additional Projects
-
-
dōTERRA[doh-ter-ah]
Video disabled by your privacy settings
-
Discover
- View Discover Home
- Healing Hands
- Product Education
- Blog
- Tools
-
doTERRA Events
 See All Events
See All Events
- Resources
- Help
Part 4: Terpene Hydrocarbons—Diterpenes
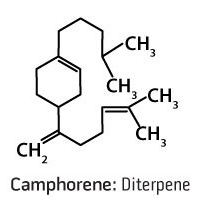
A diterepene is the combination of two monoterpene units, for a total of 20 carbons. Because of their high molecular weights, these molecules are difficult to extract by steam distillation, and when present, are found in essential oils in very low amounts. Structurally, these are the largest volatile aromatic compounds that can be collected under normal distillation conditions. Essential oil constituents typically have low molecular weights, which contributes to their high volatility. Molecules cannot be collected by steam distillation if they have a molecular mass above the low 300s. Should a molecule with a molecular mass above this amount be found in an essential oil, it would be a sign of improper extraction conditions or adulteration. They are rarely found in most essential oils. Diterpenes that may be found in essential oils include camphorene, cafestol, kahweol, cambrene, and taxideme.
-
Share
- Share on Facebook
- Tweet this
- Pin it
-
Get Link
- Hi-Res Image




