Origin: a Latin derivative
meaning "Gift of the Earth."
Part 2: Fundamental Chemistry—Bonding
Electrons are considered an atom’s most important subatomic particle because they determine the atom’s ability to undergo chemical bonding. Instead of being found within the nucleus of the atom (as with protons and neutrons), electrons are found in cloud-like layers called shells surrounding the nucleus. Imagining the function and shape of these shells can be very difficult, so it is more helpful to envision electrons as if they exist in layers. Depending on the number of electrons in an atom, it will have more layers because each layer can hold only up to eight electrons. The layers closest to the nucleus will fill up first before adding electrons in new layers farther out.
No matter how many total electrons an atom has, bonding only involves the electrons in the atom’s outermost layer, which is more commonly called the valence shell. Atoms are most stable when their valence shell is completely filled with electrons, which occurs when the valence shell has eight total electrons. In nature, molecules occur much more prevalently than single atoms because few atoms naturally have a full valence shell. In fact, only six elements (known as the noble gases) have a full valence shell and are considered so chemically stable that they do not undergo bonding. Bonding allows atoms to share, give up, or accept electrons to create a full outer shell, and in turn, increase their stability. Several types of chemical bonds exist, but only covalent bonding and ionic bonding are included within the scope of this module.
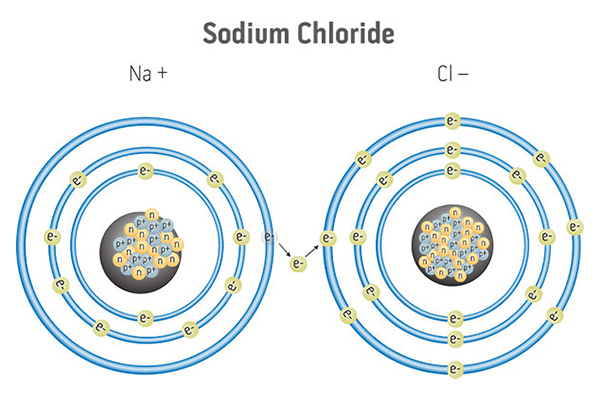
Ionic Bonding occurs when one atom “steals” a pair of electrons from another atom. This causes an overall charge imbalance because one atom now has extra electrons (net negative charge) while the other is depleted of some of its electrons (net positive charge). The opposite charges attract and cause the atoms to stick together. Some types of atoms, specifically the metals, are especially prone to ionic bonding.
When atoms bond, they form what is called a molecule. Molecules can be small, including as few as two atoms, or much larger, containing hundreds of individual atoms. Because essential oils are composed of only volatile aromatic compounds, all essential oils constituents must have a low molecular mass. Molecules cannot be collected by steam distillation if they have a molecular mass above the low 300s. Should a molecule with a molecular mass above this amount be found in an essential oil, it would be a sign of improper extraction conditions or adulteration.





